@Ben
That is really some test Ben. looking forward to more of that one.
@John Bedini
Just curious what you meant by a one summer battery.
As for where I am at. I have one battery new never had acid 14ah. Four 22ah (not from walmart!) motorcycle batteries. and Two 28ah Panasonic AGM's That has me very busy getting them cycled.
Only one of the 22ah batteries did not come to life making six batteries that are no longer a loss. So far with each charge the batteries get better. Charge faster and run longer.
This is the first time I have had more charged batteries than discharged.
usually I am always waiting for a battery to get charged for the next test. I don't know if anybody understands what I am saying, but for me this is a big deal....
It's just odd to have batteries waiting to be discharged instead of waiting to get on the charger....
Les
Announcement
Collapse
No announcement yet.
How to Make a Bedini Crystal Battery
Collapse
This is a sticky topic.
X
X
-
Ben,
That is about right and that is what I would expect to happen. The Alum battery since it was never formed it would stand .7 to 1.1 volt lower as no sulphuric acid is used. I bought one of the Mountain West charger dischargers I have some real good graphs running with this type of battery. I'm only using a load of 80 to 100Ma since that is where I'm going to run the cells. They follow a very strange charge and discharge curve. it does take a while to form the battery. What you have Ben, is a water battery because of the Hydrate in Alum, Hydrate/9 or Hydrate/6 would help on the power of the cell. I'm looking for a battery that works like Solar Lights without the problems the circuits have with the Ni-Cad's, good for one summer battery. I would form the battery very slow at 100 Ma to 300 Max until the voltage level is good. Do a C20 discharge unless you want to see if you can hurt it.
Good Work Ben.
Leave a comment:
-
See my video.....Messing around. The clerk at Advance Auto was surprised when I handed him the acid pack back!Originally posted by Lidmotor View PostThanks Ben. I am anxious the see how your new dry battery conversion graphs out. I like the fact that you just left the acid at the store. Remember that line out of that movie--"Badges? We don't need no stinking badges." Well I can just see you at the battery store---Acid? We don't need not stinking acid."
-----Lidmotor
For sure, we don't "need no stinkin badges!". For some reason, the small battery is checking out about a volt lower than the
older "Big Green Battery".... BTW, 2 Ah will boil that big battery like a teakettle when it is fully charged up around 16.5VDC. Of course
when charger removed, it drops to about 12.45 resting with full charge. Drops to about 12.1 to 12.0 with constant current 2 A load. and
does its thing.
Ben
Leave a comment:
-
Hi All,
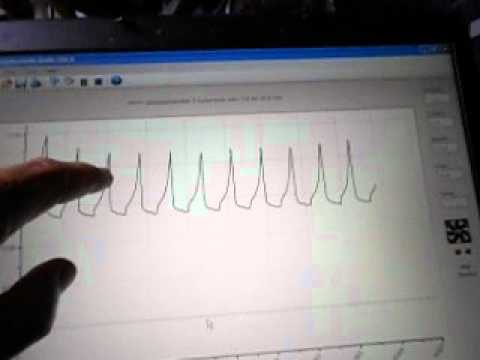
I'm doing an interesting experiment with my small 4Ah battery. It is fully charged, settled to around 10.9VDC. I'm charging it at a constant current of 200 Ma for 18 seconds, then discharging it for 18 seconds at a constant current of 200 Ma. Absolute, Square wave from pulse generator driving the SS switch. Logic would say that as no battery is 100% efficient, it should slowly discharge. The video shows what I am doing and I will post later tonight or tomorrow after a long run what happens with the battery. I am working at the top voltage of the battery in the low impedance area and going up during charge into the NON LINEAR, higher impedance mode. The waveform shows this very plainly.
It is something to think about.
Leave a comment:
-
Originally posted by Branch Gordon View PostHey all! Would this be the compound I am wanting for an alum battery conversion: Photographers' Formulary Ammonium Aluminum Sulfate - 10-0090?
yes , but they want to much
Aluminum Ammonium Sulfate 2lb Bag (Free Shipping) | eBay
Leave a comment:
-
Hey all! Would this be the compound I am wanting for an alum battery conversion: Photographers' Formulary Ammonium Aluminum Sulfate - 10-0090?
Leave a comment:
-
Thanks Ben. I am anxious the see how your new dry battery conversion graphs out. I like the fact that you just left the acid at the store. Remember that line out of that movie--"Badges? We don't need no stinking badges." Well I can just see you at the battery store---Acid? We don't need not stinking acid."
-----Lidmotor
Leave a comment:
-
Cell made from old battery negative plates
@John B. & Chuck
I was able to form up a cell using just the (-) plates that I salvaged out of that terrible old battery that I took apart. It worked just like you showed in your video. My little propeller motor will only run a few minutes but it did prove the point that you can use just the sponge lead plates and form up a rechargeable cell.
Lidmotor
Leave a comment:
-
Hi John and all,
Enjoyed your next video, I learn something new each time! I lost 35 hours of data with my big battery discharge because I forgot to set the total amount of time to chart the discharge. It quit charting @ 24 hours but
but kept discharging for 36 hours...............and going strong.........Oh well, will do another discharge after I charge it back up.
The more I play with these batteries using constant current charge and constant current discharge the more questions I get on the "Innies" and "Outies".......It takes time to show on a graph but will try to put something up tomorrow. The small battery is doing fine, needs to be cycled a few times and its working voltage is about a volt lower than the big battery but it has a very concentrated solution in it. Possibly that makes the difference in working voltage. I have not worked enough with these type of batteries to know for sure.......All, keep at it, building or testing.
Ben K4ZEP
Leave a comment:
-
Ok maybe someone had already suggested it but I believe that the missing ingredient is simply silica gel to soak up the water
Leave a comment:
-
For Europeans: E523 (E number) points to the same page.Originally posted by Retep View PostThese citations are taken from here: Ammonium aluminium sulfate - Wikipedia, the free encyclopedia
Leave a comment:
-
Hello,
I am not strong in chemistry, but may be the info here can be usable to define the hydrate gel mixture.
Cloudy Globs: Can You Make a White Gel From Two Clear Liquids? | SMILE
Best regards.
Leave a comment:
-
Ibedonc,
You take the plates from a spent gel cell battery and clean them, using the sponge lead plates only.
Leave a comment:

Leave a comment: